Acceptable levels of nitrosamines are set in nanograms and is based on what is considered as reasonably safe if a patient continues to take the affected medicine every day for a lifetime of 70 years.
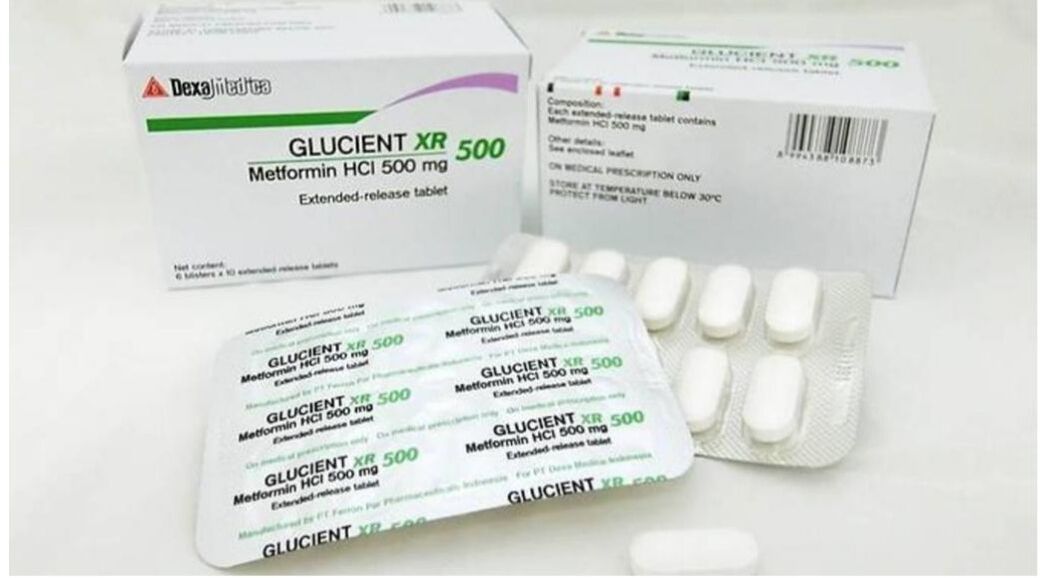
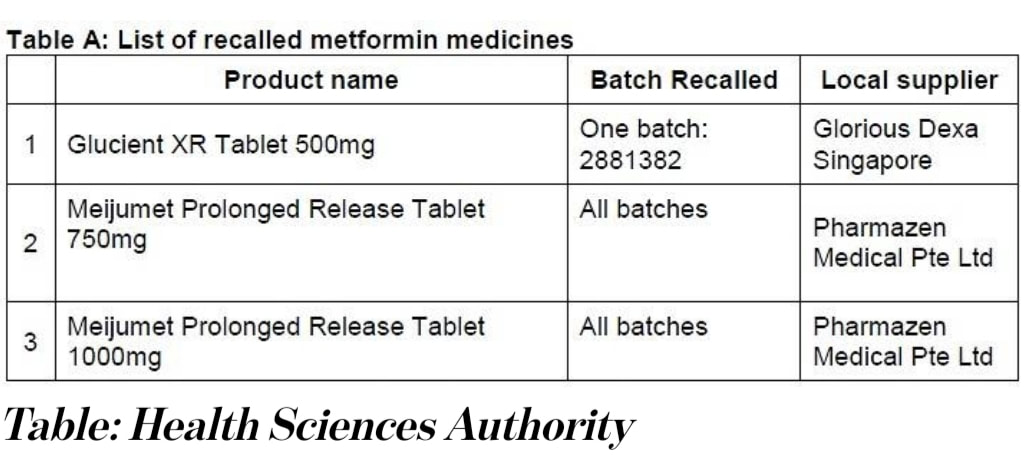
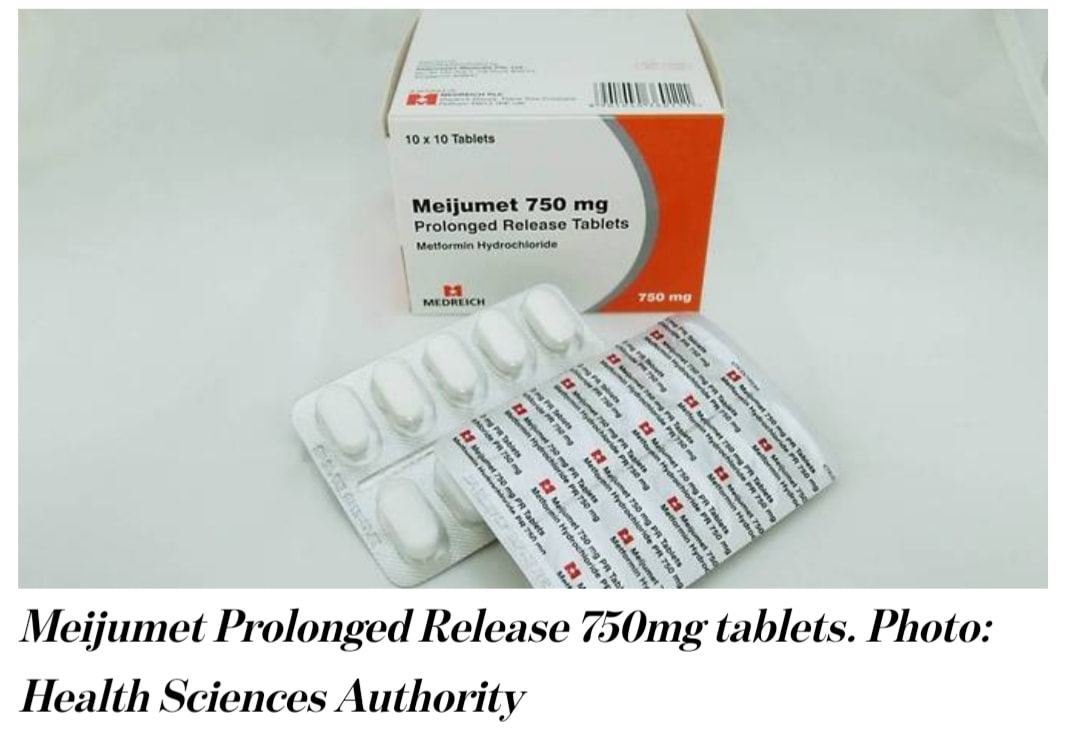
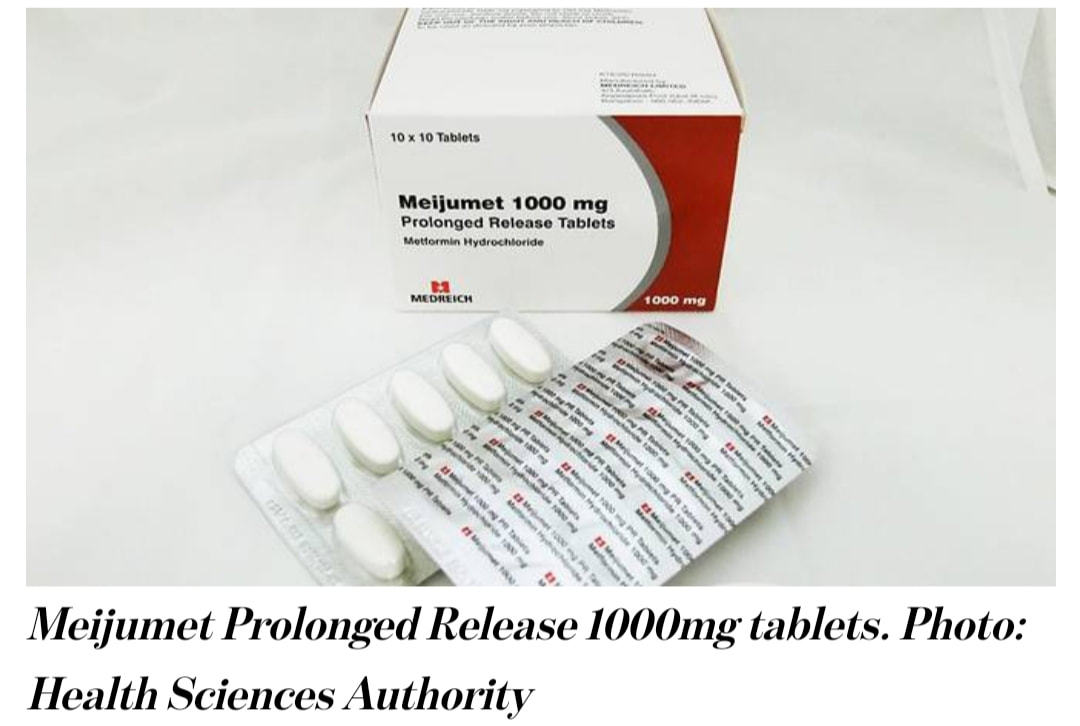
All three drugs were found to contain amounts of a type of nitrosamine impurity – known as N-nitrosodimethylamine (NDMA) – which are above the internationally acceptable level. HSA said that it has tested all 46 locally marketed metformin medicines and found that the other 43 drugs were not affected.
HSA said that it is also working with the companies supplying these medicines as well as international regulatory agencies to verify the causes of the contamination, and to address the issue.
According to HSA, worldwide recalls have been conducted for the affected products.
Healthcare professionals have been advised by HSA to contact their affected patients to arrange for an exchange of their medicines as soon as possible. Patients who are concerned about their current treatment can speak to their doctor or pharmacist.
“The additional risk posed by NDMA from metformin, at the levels detected, is considered very low,” HSA said.


 RSS Feed
RSS Feed
